The Health Inspectorate on its website publishes the information submitted by manufacturers and importers in the European Commission's database EU-CEG in the Latvian data repository submitted tobacco products, electronic cigarettes, herbal smoking products, and refilling containers under Regulation No. 440 “the procedures for providing and processing information on tobacco products, plant smoking products, electronic cigarettes and their refill vials” of the Cabinet of Ministers Paragraph 34.
The Health Inspectorate explains that, after the publication date, submitters may have made changes in the EU-CEG Latvian data repository by adding new product presentations or removing ID from the list of active products.
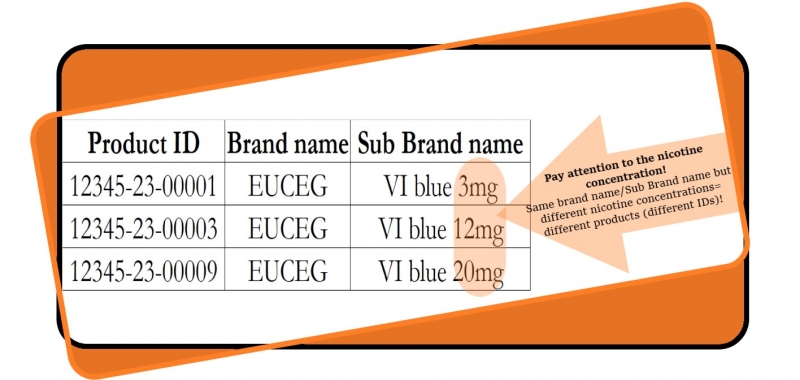
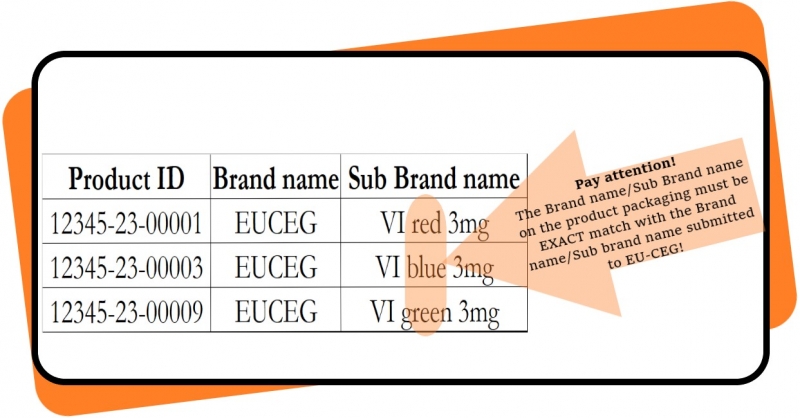
The Health Inspectorate informs that manufacturers and importers who submit information via the EU-CEG Latvian data repository are responsible for the correctness of the information submitted.
The Inspectorate informs that it is prohibited to place on the market tobacco products, electronic cigarettes, and refilling containers, if they contain prohibited substances, as follows:
- vitamins or other additives which give the impression that the tobacco product is beneficial to health or reduces health risks;
- caffeine or taurine or other additives and stimulating compounds associated with energy and vitality, containing additives that affect the color of emissions;
- additives to facilitate inhalation or intake of nicotine;
The Health Inspectorate draws attention to the fact that in future monitoring, increased attention will be paid to the requirement that prohibits such additives in products that facilitate inhalation or nicotine uptake. The list published on the website is illustrative and may be supplemented taking into account the latest scientific information. The list of ingredients available according to current scientific knowledge:
- additives that have carcinogenic, mutagenic, and toxic properties for reproduction.
Only such ingredients are used in the nicotine-containing or nicotine-free liquid which does not pose a risk to human health in heated or unheated form. This Clause shall not apply to nicotine.
The Inspectorate informs that it is prohibited to place on the market tobacco products, plant smoking products, electronic cigarettes, refilling containers, and novel tobacco products, regarding which information has not been submitted under Section 5, Paragraphs one and two of the Law on the Circulation of Tobacco Products, Plant smoking products, electronic smoking devices, and their liquids (hereinafter - Tobacco Law)
Based on the requirements specified in Section 5, Paragraph One of the Law, manufacturers and importers shall make a fee for the processing of the information provided regarding tobacco products, electronic cigarettes, and filling containers under the price list of the Health Inspectorate charge services.
For more information about fees and information and technical support follow the link.
According to Section 3, Paragraph One of the Tobacco Law, it is prohibited to market electronic cigarettes and refill containers for which the manufacturer or importer has not performed the submission of information and payment for the processing of information.
Electronic cigarettes and refill containers are allowed to be placed on the market for six months after their notification of EU-CEG.
The Health Inspectorate informs that the Excel file contains the products submitted in the EU-CEG Latvian Data Repository for which payment has been made under Section 5, Paragraph One of the Tobacco Law find it here.
Information is updated once a month.